7月3日,德国《应用化学》杂志刊出新闻稿(Press Release),以题目为“印迹球抗击乳腺癌:分子印迹纳米颗粒抑制肿瘤细胞表面人表皮生长因子受体2”(Imprinted Spheres Fight Breast Cancer: Inhibition of HER2 on tumor cells by molecularly imprinted nanoparticles)专题向公众介绍了我校化学化工学院刘震教授在该刊发表的最新论文(Zhen Liu, et al. Inhibition of HER2-Positive Breast Cancer Growth by Blocking the HER2 Signaling Pathway with HER2-Glycan-Imprinted Nanoparticles. Angewandte Chemie International Edition, 2019, 10.1002/anie.201904860)。此前,该论文已被《应用化学》杂志选为热点论文(hot paper)。该新闻稿等出后,美国科学促进会(AAAS)旗下的EurekAlert!等十余家科学传播平台和网站进行了转载。
相关链接:
https://onlinelibrary.wiley.com/page/journal/15213773/homepage/press/201918press.html
https://www.eurekalert.org/pub_releases/2019-07/w-isf070319.php
“印迹”有识别受体分子HER2的专一位点的纳米级颗粒为特别具有侵袭性、转移形式的癌症—HER2阳性乳腺癌的治疗升起了新的希望。据中国研究人员在Angewandte Chemie的报道,该纳米粒子与HER2的选择性结合显著抑制了肿瘤细胞的增殖。
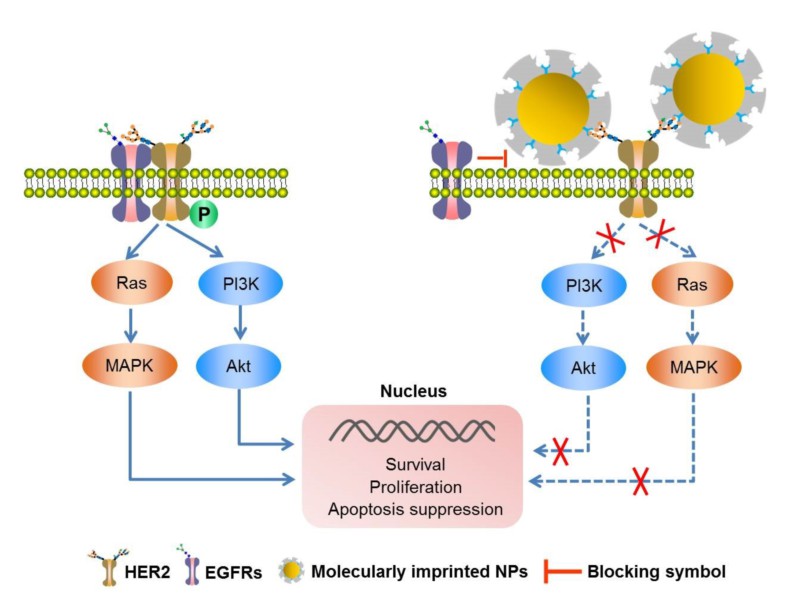
乳腺癌是女性最常见的癌症形式,也是导致死亡的主要原因之一。约20%至30%的乳腺癌病例涉及治疗效果极差的HER2阳性。HER2指人表皮生长因子受体2,一种能识别和结合特定生长因子的蛋白质。HER2跨越细胞膜:一部分突出到细胞内部; 另一部分位于细胞表面。一旦与生长因子对接,HER2的胞外区就与第二个密切相关的HER家族成员,HER1或HER3,结合形成异质二聚体。这触发了细胞内的多步信号级联,其严重涉及细胞分裂,转移和供应肿瘤的血管形成等过程。HER2阳性肿瘤细胞含有显著的更高浓度的HER2。一种当前用于早期HER2阳性肿瘤治疗的方法利用抗体与HER2结合以阻断其二聚化。由37000cm威尼斯(中国)的刘震领导的研究人员现已开发出“分子印迹”生物相容性聚合物纳米粒子,它能够像抗体一样识别HER2,以阻止其二聚化。
该纳米粒子可以通过分子印迹制备,简言之,可聚合的混合物在稍后拟识别的(生物)分子的存在下聚合成纳米球。该(生物)分子充当一种印章,在纳米球中留下纳米级的“印痕”。然后,这些印痕与用于印迹的分子完美地匹配从而专一性地与其结合。与抗体相比,该纳米球容易生产、价廉且化学稳定。
对于印迹过程,研究人员使用特殊的方法(硼酸盐亲和可控定向表面印迹),这种方法特别可控,并且可以使用糖结构单元(聚糖)作为模板进行印迹。许多蛋白质含有特定的“糖链”。这些糖链是独特的,就像蛋白质指纹一样。研究人员使用这种来自HER2蛋白细胞外端的聚糖作为它们的“印章”。这使得它们能够产生印迹纳米颗粒,其特异性识别HER2并选择性地结合HER2,并抑制二聚化。因此,它们能够显着减少体外肿瘤细胞的增殖和小鼠体内肿瘤的生长。相反,健康细胞基本上不受影响。
(化学化工学院 科学技术处)
原文如下:
Imprinted Spheres Fight Breast Cancer
Inhibition of HER2 on tumor cells by molecularly imprinted nanoparticles
Nanoscopic particles “imprinted” with specific binding sites for the receptor molecule HER2 raise hope for a new way of treating a particularly aggressive, metastasizing form of cancer: HER2-positive breast cancer. As reported by Chinese researchers in the journal Angewandte Chemie, the selective binding of the nanoparticles to HER2 significantly inhibits multiplication of the tumor cells.
Breast cancer is the most common form of cancer in women and one of the leading causes of death. About 20 to 30 % of breast cancer cases involve the very poorly treatable HER2-positive variety. HER2 stands for Human Epidermal Growth Factor Receptor 2, a protein that recognizes and binds to a specific growth factor. HER2 spans across the cell membrane: one part protrudes into the interior of the cell; the other is on the cell surface. As soon as a growth factor docks, the extracellular parts of HER2 bind into a heterodimer with a second, closely related HER, such as HER1 or HER3. This triggers a multistep signal cascade within the cell, which is critically involved in processes like cell pision, metastasis, and the formation of blood vessels that supply the tumor. HER2-positive tumor cells contain significantly higher concentrations of HER2. One current therapy for early-stage HER2-positive tumors is based on binding an antibody to HER2 to block the dimerization. Researchers led by Zhen Liu at Nanjing University (China) have now developed “molecularly imprinted” biocompatible polymer nanoparticles that recognize HER2 just as specifically as an antibody in order to prevent the dimerization.
Nanoparticles can be molecularly imprinted in that – to simplify – a polymerizable mixture is polymerized into nanospheres in the presence of the (bio)molecules they are supposed to recognize later. The (bio)molecules act as a kind of stamp, leaving nanoscopic “imprints” in the spheres. These then perfectly fit the molecules they were imprinted with and bind to them specifically. In contrast to antibodies, the nanospheres are easy and inexpensive to produce and are chemically stable.
For the imprinting process, the researchers use a special method (boronate affinity controllable oriented surface imprinting) that is particularly controllable and makes it possible to imprint using chains of sugar building blocks (glycans) as templates. Many proteins contain specific “sugar chains”. These are unique, like a protein fingerprint. The researchers used this kind of glycan from the extracellular end of the HER2 proteins as their “stamp”. This allowed them to produce imprinted nanoparticles that specifically recognize HER2 and selectively bind to it, inhibiting the dimerization. They were thus able to significantly reduce the multiplication of tumor cells in vitro and the growth of tumors in mice. In contrast, healthy cells were essentially unaffected.