Recently, Professor Shi Yi, Li Songlin and their teams from the School of Electronic Science and Engineering made new progress in dimensionally confined semiconductors and published a research paper titled “Oxidation kinetics and non-Marcusian charge transfer in dimensionally confined semiconductors” on Nature Communications (https://doi.org/10.1038/s41467-023-39781-y). The paper has three joint first authors: Xu Ning, a Ph.D. student from the School of Electronics Engineering; Shi Li, a doctoral student from the School of Physics at Southeast University (currently an Associate Professor at Nanjing University of Posts and Telecommunications); and Pei Xudong, a Ph.D. student from the School of Modern Engineering. The corresponding authors of the paper are Associate Professor Li Songlin, Professor Shi Yi, Professor Wang Peng from the School of Modern Engineering, and Professor Wang Jinlan from the School of Physics at Southeast University. The research work received strong support from Professor Chen Jian of the School of Electronics Engineering.
The abstract of the paper is as following:
Electrochemical reactions represent essential processes in fundamental chemistry that foster a wide range of applications. Although most electrochemical reactions in bulk substances can be well described by the classical Marcus-Gerischer charge transfer theory, the realistic reaction character and mechanism in dimensionally confined systems remain unknown. Here, we report the multiparametric survey on the kinetics of lateral photooxidation in structurally identical WS2 and MoS2 monolayers, where electrochemical oxidation occurs at the atomically thin monolayer edges. The oxidation rate is correlated quantitatively with various crystallographic and environmental parameters, including the density of reactive sites, humidity, temperature, and illumination fluence. In particular, we observe distinctive reaction barriers of 1.4 and 0.9 eV for the two structurally identical semiconductors and uncover an unusual non-Marcusian charge transfer mechanism in these dimensionally confined monolayers due to the limit in reactant supplies. A scenario of band bending is proposed to explain the discrepancy in reaction barriers. These results add important knowledge into the fundamental electrochemical reaction theory in low-dimensional systems.
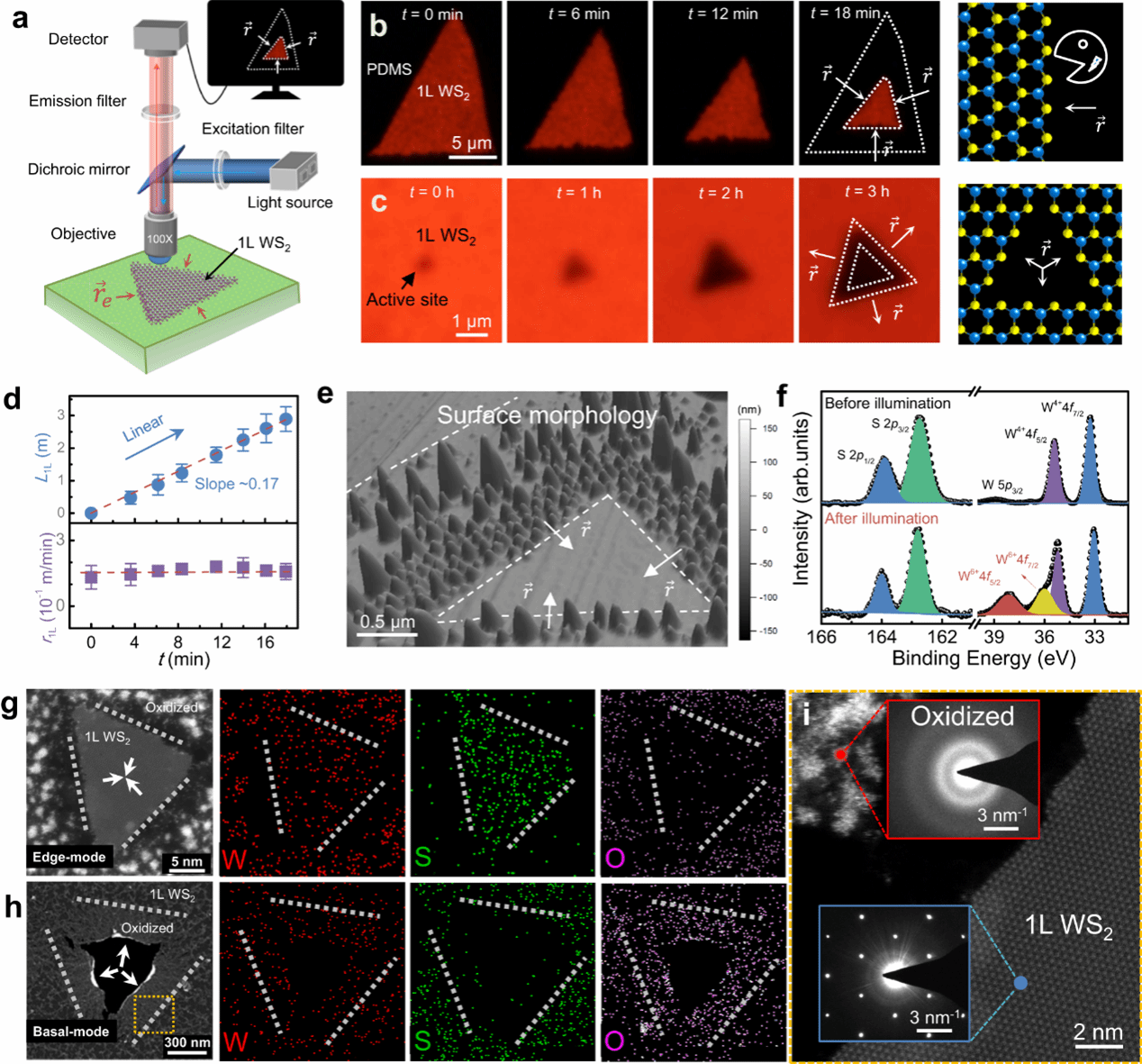
Fig. 1: Photoluminescence-traced photo-oxidation process in monolayer (1 L) WS2 .
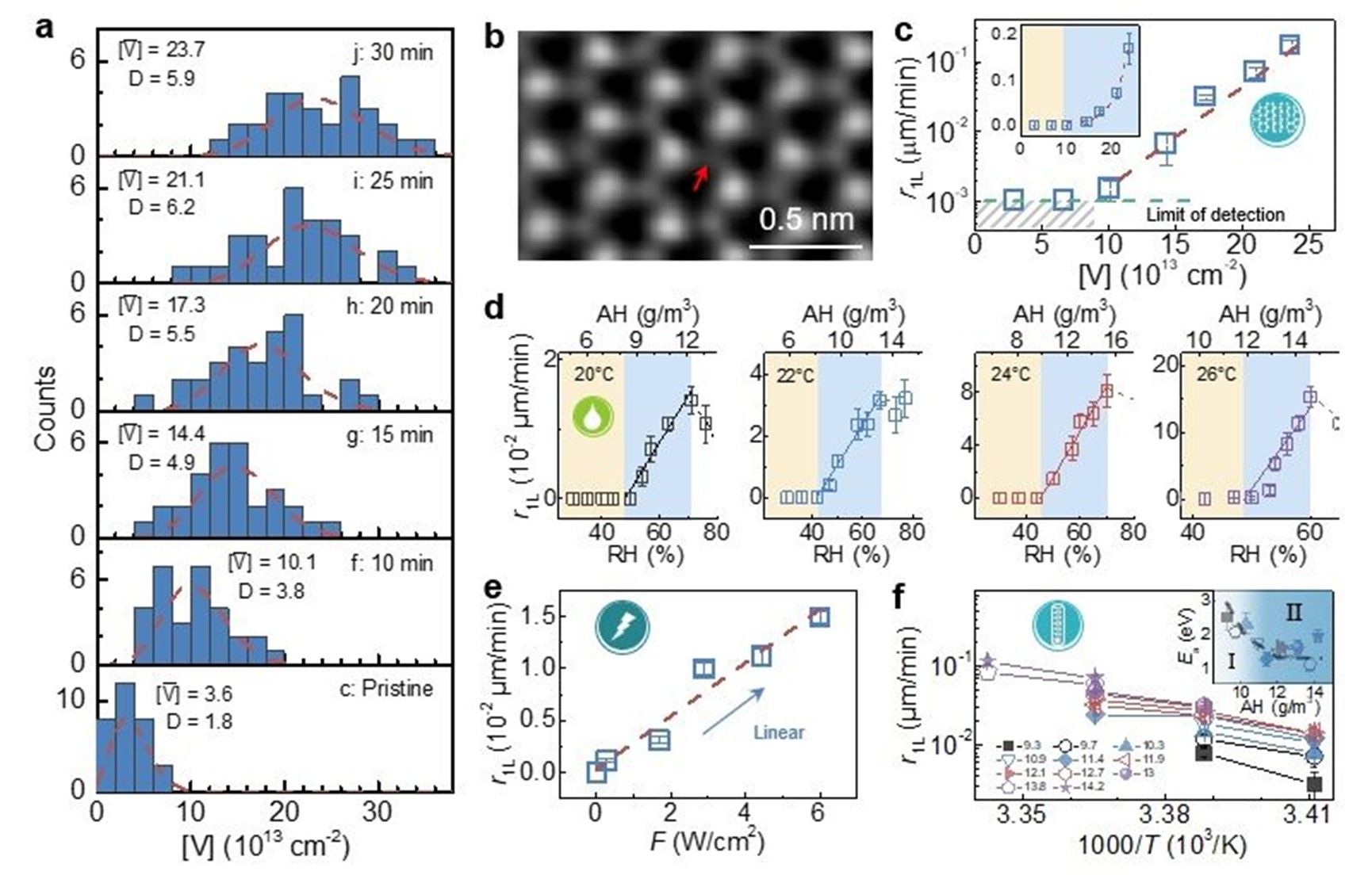
Fig. 2: Multiparametric survey on oxidation kinetics in 1L WS2 .
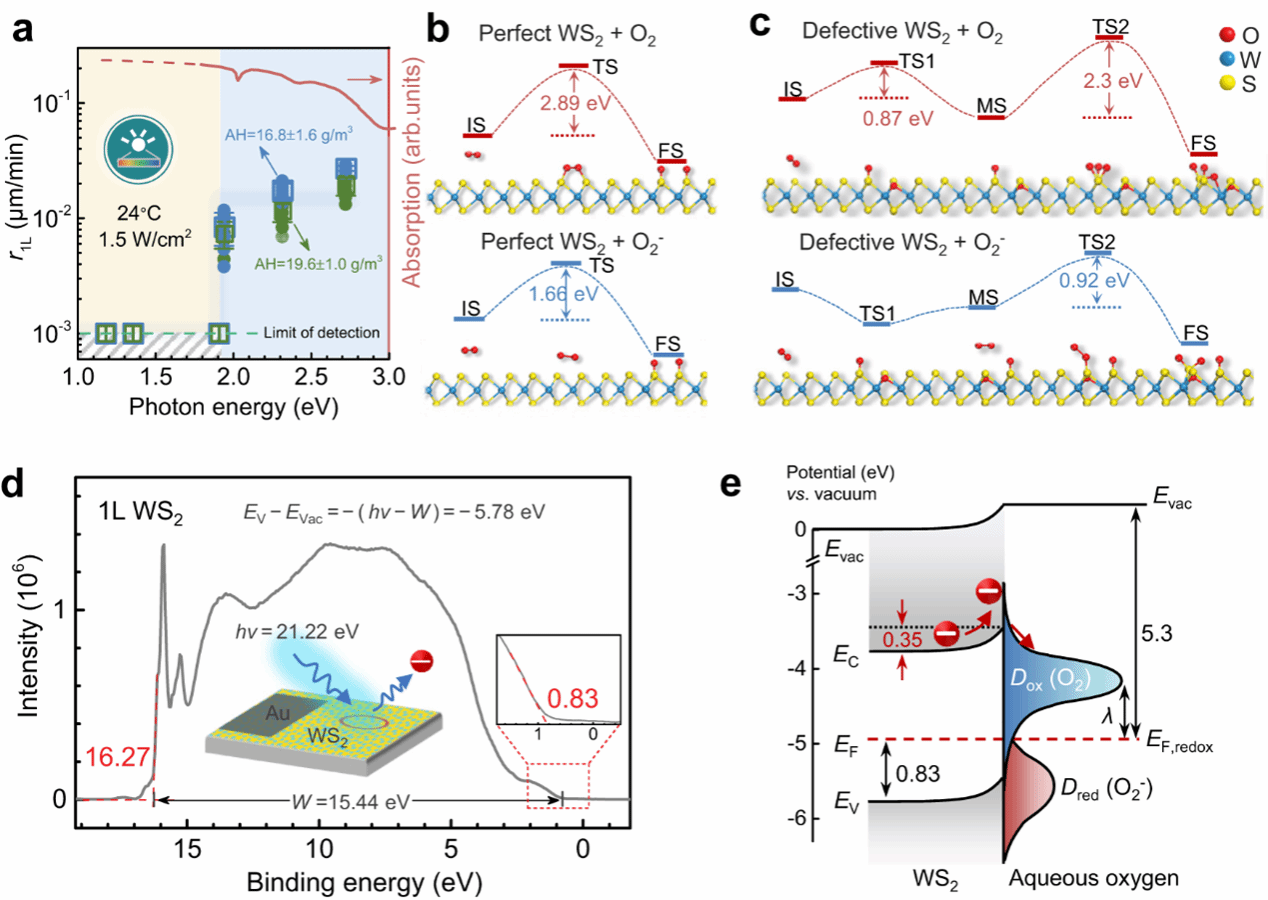
Fig. 3: Microscopic oxidation mechanisms with simulated reaction paths and derived band alignment.
Editors: Guo Ankang, Li Jiesheng
