Recently, Professor Xu Qiang and his team from the School of Life Sciences made new progress in interleukin and published a research paper titled “An autonomous activation of interleukin-17 receptor signaling sustains inflammation and promotes disease progression” on Immunity (https://doi.org/10.1016/j.immuni.2023.06.012). Professor Xu is the corresponding author of this paper, and Research Associate Luo Qiong, also from the School of Life Sciences, is the first author and co-corresponding author.
The abstract of the paper is as following:
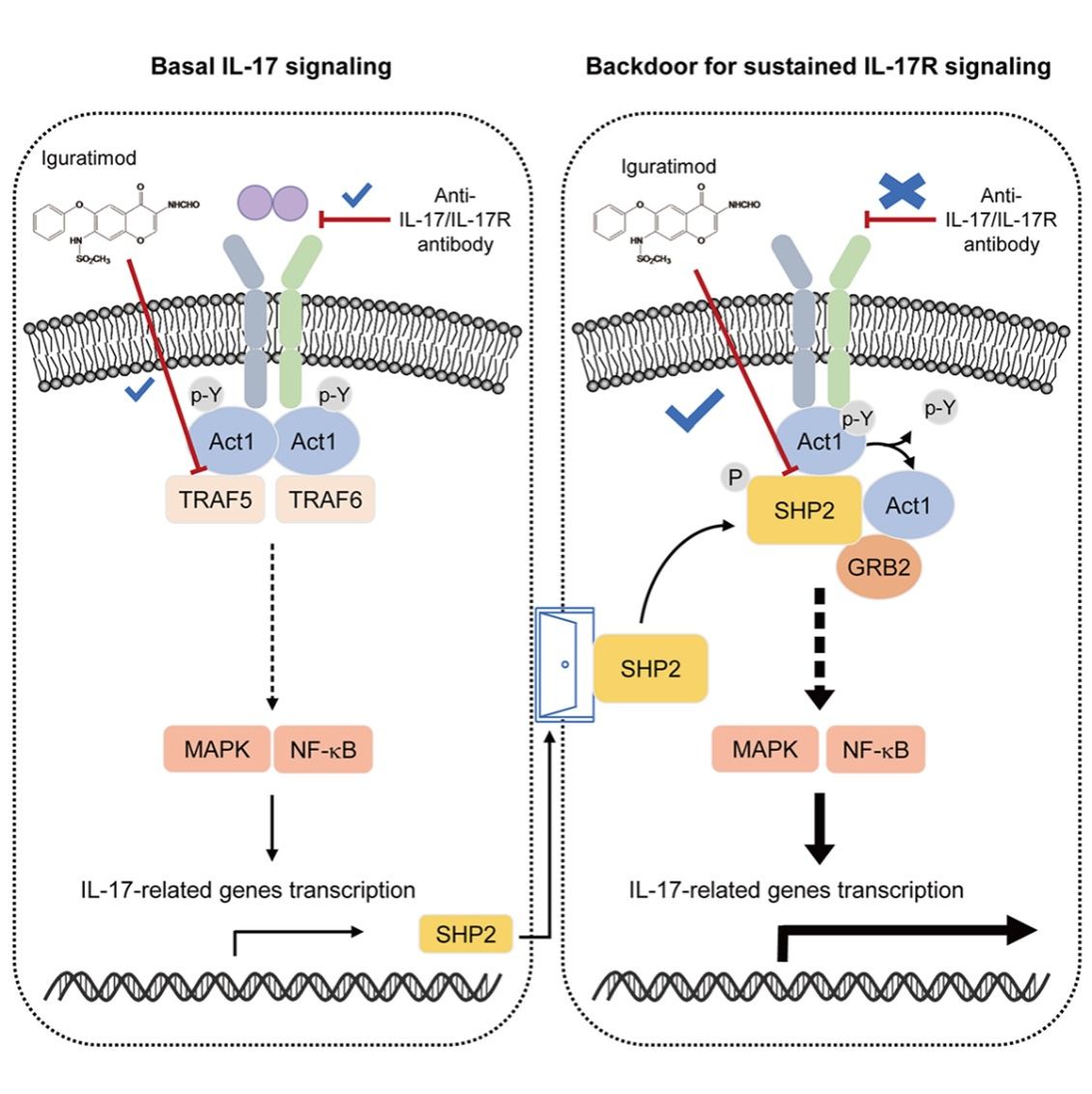
Anti-interleukin-17 (IL-17) therapy has been used in various autoimmune diseases. However, the efficacy is unexpectedly limited in several IL-17-associated diseases, and the mechanism of limited efficacy remains unclear. Here, we show that a molecular complex containing the adaptor molecule Act1 and tyrosine phosphatase SHP2 mediated autonomous IL-17R signaling that accelerated and sustained inflammation. SHP2, aberrantly augmented in various autoimmune diseases, was induced by IL-17A itself in astrocytes and keratinocytes, sustaining chemokine production even upon anti-IL-17 therapies. Mechanistically, SHP2 directly interacted with and dephosphorylated Act1, which replaced Act1-TRAF5 complexes and induced IL-17-independent activation of IL-17R signaling. Genetic or pharmacologic inactivation of SHP2, or blocking Act1-SHP2 interaction, paralyzed both IL-17-induced and IL-17-independent signaling and attenuated primary or relapsing experimental autoimmune encephalomyelitis. Therefore, Act1-SHP2 complexes mediate an alternative pathway for autonomous activation of IL-17R signaling, targeting which could be a therapeutic option for IL-17-related diseases in addition to current antibody therapies.
Editors: Guo Ankang, Li Jiesheng
